Organization
At T-CReDO, teams are organized by function and role within a matrix-style project management framework, enabling the delivery of seamless support.
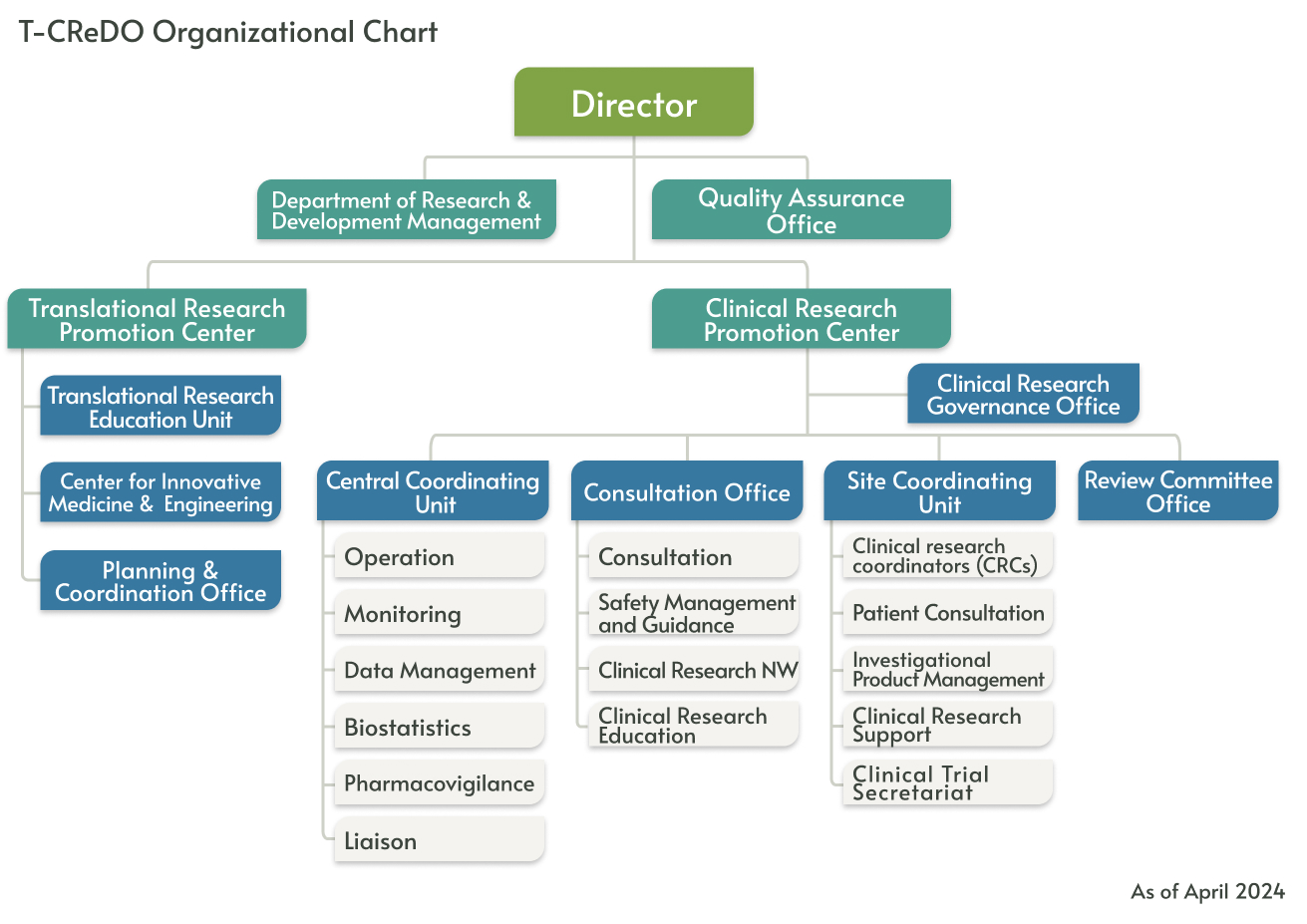
Department of Research & Development Management
The Department of Research & Development Management provides one-stop service that covers the complete spectrum from clinical medical research development to practical implementation. It conducts research and development management, including medical seed pipeline management.
To facilitate the commercialization of early-stage discoveries, they offer comprehensive support including intellectual property counseling, regulatory consulting, partnerships with companies and clinicians, evaluation and selection through AMED's translational research programs, research funding support, and contract management.
Quality Assurance Office
Since 2016, the Quality Assurance Office has continuously supported clinical trials and has managed audit operations. This office assesses trial processes and systems —including regulatory compliance, protocol, compliance with standard operations procedures (SOPs*1), informed consent forms (ICF*2), case report forms (CRF*3), and trial management procedures— to verify appropriate implementation.
Audit objective is to verify that clinical trials and research comply with regulations, guidelines, protocols, and SOPs for quality assurance, operating independently from routine monitoring and quality control functions.
*1: A set of written instructions that document a routine or repetitive activity followed by an organization. SOPs are designed to ensure consistency and quality in the performance of specific functions.
*2: A document that provides potential research participants with information about the study, including its purpose, duration, required procedures, potential risks, and benefits. It ensures that participants can make an informed decision about their involvement.
*3: A specialized document used to collect data from each participant in a clinical trial. CRFs are designed to capture all the necessary information related to the study's objectives and regulatory requirements.
Translational Research Promotion Center

This department has three main objectives: To bring research concepts to life, to develop the next generation of clinical research professionals, and to drive commercialization to accelerate the translation of research into clinical practice.
Translational Research Education Unit
Offers professional training programs to develop global talent for international commercialization of medical startups through:
- Research Studio platform :
Promotes medical entrepreneurship through inter-university collaboration, social implementation of academic research, and startup development support for medical seeds. - Business Education Program :
Human resource development for effective management and business growth. - Regulatory Science :
Regulatory pathways education for new drugs and new medical devices.
Center for Innovative Medicine and Engineering (CIME)
Supports clinical feasibility studies to facilitate the clinical adoption of medical devices and pharmaceuticals developed through interdisciplinary research, especially from medical-engineering collaboration. Since its strategic establishment within the hospital in January 2014, 17 projects have leveraged the CIME validation research space to conduct physician-initiated clinical trials and specific clinical research over nine years. The center underwent a major renewal in August 2024, expanding it’s capabilities with a new shared office, incubation support services, and enhanced medical-engineering collaboration support. It also hosts seminars and workshops for university and external researchers interested in medical device development, fostering cross-disciplinary researcher exchange.
Planning & Coordination Office
This department works with internal and external stakeholders to achieve the vision and objectives of the Translational Research Promotion Center. They plan, coordinate, and drive various activities to strengthen research support, promote industry-academia partnerships, and develop projects that advance translational research.
Clinical Research Promotion Center

Promotes clinical trials and clinical research while ensuring the protection of research participants and maintaining research integrity.
Clinical Research Governance Office
Develops and oversees procedures to ensure clinical research at our hospital follows all regulations and is conducted properly. When compliance issues arise, they provide consultation and guidance on reporting procedures and prevention strategies. As the secretariat for the Specified Clinical Research Management Committee, they support the hospital director in centrally managing all clinical research activities and help strengthen the governance framework.
Central Coordinating Unit
Manages investigator-initiated clinical trials with specialists who have industry R&D experience. It ensures high-quality trials through development strategy, protocol creation, data management, monitoring, and statistical analysis. It also leverages AI technology for efficiency and collaborates with external AROs to enhance support capabilities. Additionally, it builds and manages EDC (Electronic Data Capture) systems for specified clinical research to ensure reliable data collection.
Consultation Office
Supports the planning of clinical research, primarily based on the Clinical Research Act, and offers a variety of consultation services related to clinical research. It offers ethics training and seminars to support the professional development of researchers from within the university and the broader community.It promotes collaboration with other universities through the National University Hospital, Clinical Research Promotion Initiative and the University Hospital Clinical Trial Alliance. Within our hospital, it facilitates information sharing with representatives from all clinical departments through regular liaison meetings.
Site Coordinating Unit
Clinical Research Coordinators (CRCs) work with physicians and coordinate across departments to ensure the smooth implementation of trials and support participant involvement. Investigational Product Pharmacists ensure the proper storage, management, and dispensing of investigational products to maintain quality and safety. The Clinical Trial Secretariat handles site selection, contracting, and administrative tasks from preparation through execution. The clinical research support division provides monitoring services, including progress tracking, quality control, and the development of optimal monitoring plans using a Risk-Based Approach (RBA).
Review Committee Office
Serves as the secretariat for multiple committees including the Institutional Review Board, Clinical Research Review Board, Ethical Committee, Certified Special Committee for Regenerative Medicine, and Conflict of Interest Committee. This office coordinates with various departments and responds flexibly to sponsor and researcher requests to ensure fair and efficient operations. They also support the Unapproved Drug Clinical Use Evaluation Committee, which reviews ethical and scientific validity of unapproved drugs and devices before clinical use.






